If you're interested in becoming a contributor or requesting changes then click here to join the discord
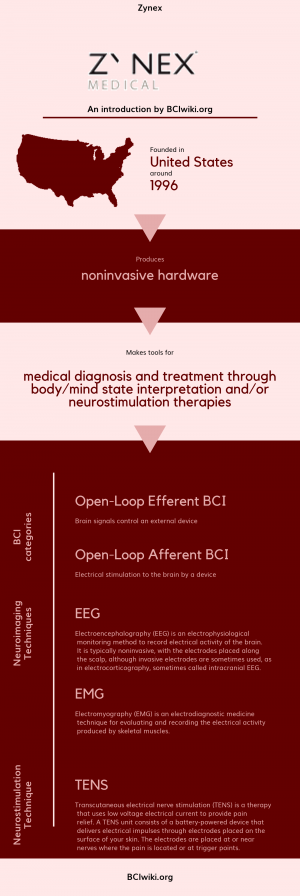
Zynex
"Zynex, Inc. founded in 1996, engineers, manufactures and markets neuro-diagnostics, stroke rehabilitation, pain management and cardiac monitoring devices within three subsidiaries. Zynex Medical is a provider of electrotherapy products for home use. Zynex Monitoring Solutions develops products for cardiac monitoring for use in hospitals. Zynex NeuroDiagnostics develops devices for EMG and EEG diagnostic applications within neurology disciplines."
Founded in The United States around 1996, Zynex produces noninvasive hardware.
Zynex makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent, Open-Loop Afferent
Neurosensing Technique(s): EEG, EMG
Neurostimulation Technique(s): TENS
FDA
Zynex has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K191697 | Plethysmograph, Impedance | 2 | Cardiovascular | False | True |