If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Magstim"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| − | [[Category: | + | [[Category:Journals]] |
"Magstim is a supplier of Transcranial Magnetic Stimulation (TMS) stimulators and packages used for Magstim TMS therapy." | "Magstim is a supplier of Transcranial Magnetic Stimulation (TMS) stimulators and packages used for Magstim TMS therapy." | ||
Magstim is the child company of [[Welcony]] | Magstim is the child company of [[Welcony]] | ||
| + | |||
[[File:Magstim.png|thumb|Magstim Company Profile]] | [[File:Magstim.png|thumb|Magstim Company Profile]] | ||
| Line 16: | Line 17: | ||
[[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: TMS | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: TMS | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BiStim2|BiStim2]] | ||
| + | *[[D110_Cone_Coil|D110 Cone Coil]] | ||
| + | *[[D25_Alpha_BI_Coil|D25 Alpha BI Coil]] | ||
| + | *[[D40_Alpha_Flat_Coil|D40 Alpha Flat Coil]] | ||
| + | *[[D50_Alpha_Flat_Coil|D50 Alpha Flat Coil]] | ||
| + | *[[D60_Alpha_Flat_Coil|D60 Alpha Flat Coil]] | ||
| + | *[[D70_Air_Film_Coil|D70 Air Film Coil]] | ||
| + | *[[D70_Alpha_Coil|D70 Alpha Coil]] | ||
| + | *[[D70_Alpha_Flat_Coil|D70 Alpha Flat Coil]] | ||
| + | *[[D70_MT_Coil|D70 MT Coil]] | ||
| + | *[[D70_Remote_Coil|D70 Remote Coil]] | ||
| + | *[[D70%C2%B2_Coil|D70%C2%B2 Coil]] | ||
| + | *[[Magstim_2002|Magstim 2002]] | ||
| + | *[[Rapid2|Rapid2]] | ||
| + | *[[S90_Remote_Coil|S90 Remote Coil]] | ||
| + | *[[S90_Standard_Coil|S90 Standard Coil]] | ||
| + | ==FDA== | ||
| + | Magstim has 9 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Magstim_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K223154 K223154] || Transcranial Magnetic Stimulator || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K203684 K203684] || Neurosurgical Nerve Locator || 2 || Ear, Nose, Throat || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K180907 K180907] || Transcranial Magnetic Stimulator || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K222171 K222171] || Transcranial Magnetic Stimulator || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K181559 K181559] || Neurosurgical Nerve Locator || 2 || Ear, Nose, Throat || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.magstim.com/ Website] | [https://www.magstim.com/ Website] | ||
[https://www.crunchbase.com/organization/the-magstim-company Crunchbase] | [https://www.crunchbase.com/organization/the-magstim-company Crunchbase] | ||
[https://www.linkedin.com/company/the-magstim-company-limited/ LinkedIn] | [https://www.linkedin.com/company/the-magstim-company-limited/ LinkedIn] | ||
[https://twitter.com/magstimTMS Twitter] | [https://twitter.com/magstimTMS Twitter] | ||
Latest revision as of 02:03, 18 April 2023
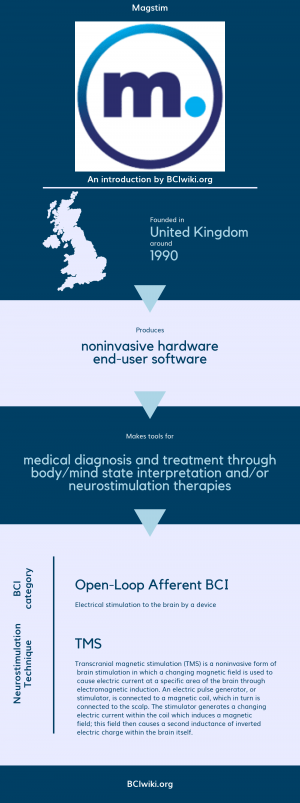
"Magstim is a supplier of Transcranial Magnetic Stimulation (TMS) stimulators and packages used for Magstim TMS therapy."
Magstim is the child company of Welcony
Founded in The United Kingdom around 1990, Magstim produces noninvasive hardware and end-user software.
Magstim makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): TMS
Hardware
- BiStim2
- D110 Cone Coil
- D25 Alpha BI Coil
- D40 Alpha Flat Coil
- D50 Alpha Flat Coil
- D60 Alpha Flat Coil
- D70 Air Film Coil
- D70 Alpha Coil
- D70 Alpha Flat Coil
- D70 MT Coil
- D70 Remote Coil
- D70%C2%B2 Coil
- Magstim 2002
- Rapid2
- S90 Remote Coil
- S90 Standard Coil
FDA
Magstim has 9 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K223154 | Transcranial Magnetic Stimulator | 2 | Neurology | False | True |
| K203684 | Neurosurgical Nerve Locator | 2 | Ear, Nose, Throat | False | True |
| K180907 | Transcranial Magnetic Stimulator | 2 | Neurology | False | True |
| K222171 | Transcranial Magnetic Stimulator | 2 | Neurology | False | True |
| K181559 | Neurosurgical Nerve Locator | 2 | Ear, Nose, Throat | False | True |