If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "NeuroWave"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) (Created page with "Category:Organizations Category:Companies We are an ISO 13485 medical device company, dedicated to developing innovative, state-of-the-art signal processing technolog...") |
Landonodnal (talk | contribs) |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Category:LinkedIn Accounts]] | ||
| + | [[Category:GitHub Accounts]] | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| − | + | We are an ISO 13485 medical device company, dedicated to developing innovative, state-of-the-art signal processing technologies for the next generation of brain monitors for improved and safer patient care. | |
| + | |||
| + | |||
| + | [[File:NeuroWave.png|thumb|NeuroWave Company Profile]] | ||
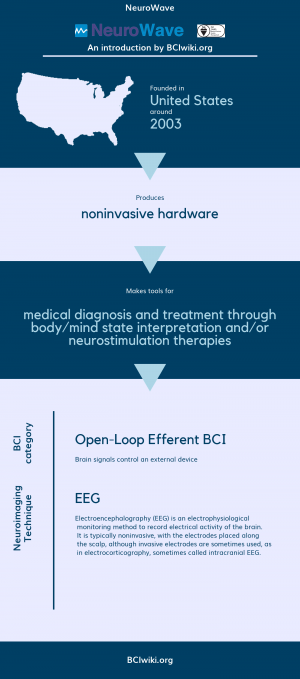
| + | Founded in The United States around 2003, NeuroWave produces noninvasive hardware. | ||
| + | |||
| + | NeuroWave makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies. | ||
| + | |||
| + | [[Brain Computer Interface Classification|BCI Categories]]: Open-Loop Efferent | ||
| + | |||
| + | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[EasyPrep_EK-901_Electrodes|EasyPrep EK-901 Electrodes]] | ||
| + | *[[NeuroSENSE_NS-901_Monitor|NeuroSENSE NS-901 Monitor]] | ||
| + | *[[NeuroSENSE_OEM_Module|NeuroSENSE OEM Module]] | ||
| + | ==FDA== | ||
| + | NeuroWave has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[NeuroWave_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K202621 K202621] || Index-Generating Electroencephalograph Software || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K142834 K142834] || Non-Normalizing Quantitative Electroencephalograph Software || 2 || Neurology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[http://www.neurowavesystems.com/ Website] | [http://www.neurowavesystems.com/ Website] | ||
| + | [https://www.crunchbase.com/organization/neurowave Crunchbase] | ||
| + | [https://www.linkedin.com/company/neurowave-systems-inc-/ LinkedIn] | ||
| + | [https://github.com/neurowavesys GitHub] | ||
Latest revision as of 02:25, 18 April 2023
We are an ISO 13485 medical device company, dedicated to developing innovative, state-of-the-art signal processing technologies for the next generation of brain monitors for improved and safer patient care.
Founded in The United States around 2003, NeuroWave produces noninvasive hardware.
NeuroWave makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EEG
Hardware
FDA
NeuroWave has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K202621 | Index-Generating Electroencephalograph Software | 2 | Neurology | False | True |
| K142834 | Non-Normalizing Quantitative Electroencephalograph Software | 2 | Neurology | False | True |