If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Syntermed"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 14: | Line 14: | ||
[[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: PET | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: PET | ||
| + | ==FDA== | ||
| + | Syntermed has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Syntermed_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K180077 K180077] || System, Tomography, Computed, Emission || 2 || Radiology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K223422 K223422] || System, Tomography, Computed, Emission || 2 || Radiology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.syntermed.com/ Website] | [https://www.syntermed.com/ Website] | ||
[https://www.crunchbase.com/organization/syntermed Crunchbase] | [https://www.crunchbase.com/organization/syntermed Crunchbase] | ||
[https://www.linkedin.com/company/syntermed-inc/ LinkedIn] | [https://www.linkedin.com/company/syntermed-inc/ LinkedIn] | ||
[https://twitter.com/SyntermedInc Twitter] | [https://twitter.com/SyntermedInc Twitter] | ||
Revision as of 02:43, 18 April 2023
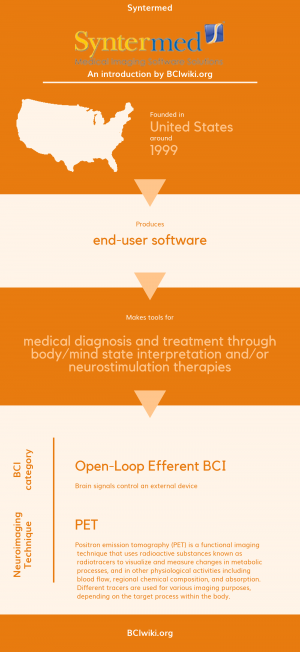
"Syntermed is a leading provider of nuclear imaging software. We establish alliances with developers, medical centers, and other medical software companies for the purpose of enhancing our product line and the services. Our goal is to be able to offer a complete line of medical imaging software products encompassing all medical imaging modalities."
Founded in The United States around 1999, Syntermed produces end-user software.
Syntermed makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): PET
FDA
Syntermed has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K180077 | System, Tomography, Computed, Emission | 2 | Radiology | False | True |
| K223422 | System, Tomography, Computed, Emission | 2 | Radiology | False | True |