If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Boston Scientific"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Category:GitHub Accounts]] | ||
| + | [[Category:LinkedIn Accounts]] | ||
| + | [[Category:Facebook Pages]] | ||
| + | [[Category:Twitter Accounts]] | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| Line 10: | Line 14: | ||
[[Brain Computer Interface Classification|BCI Categories]]: Open-Loop Afferent | [[Brain Computer Interface Classification|BCI Categories]]: Open-Loop Afferent | ||
| + | |||
| + | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: DBS, SCS | ||
| + | ==FDA== | ||
| + | Boston Scientific has 3428 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Boston_Scientific_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P130030 P130030] || Stent, Coronary || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P900056 P900056] || Catheter, Coronary, Atherectomy || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P920047 P920047] || Cardiac Ablation Percutaneous Catheter || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P010012 P010012] || Defibrillator, Automatic Implantable Cardioverter, With Cardiac Resynchronization (Crt-D) || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P920023 P920023] || Stent, Urethral, Bulbous, Permanent Or Semi-Permanent || 3 || Gastroenterology, Urology || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K163174 K163174] || Catheters, Transluminal Coronary Angioplasty, Percutaneous || 2 || Cardiovascular || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K191446 K191446] || Stent, Ureteral || 2 || Gastroenterology, Urology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K203322 K203322] || Dislodger, Stone, Biliary || 2 || Gastroenterology, Urology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K170815 K170815] || Analyzer, Pacemaker Generator Function || 2 || Cardiovascular || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K133700 K133700] || Stents, Drains And Dilators For The Biliary Ducts || 2 || Gastroenterology, Urology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[http://www.bostonscientific.com/en-US/Home.html Website] | [http://www.bostonscientific.com/en-US/Home.html Website] | ||
[https://www.crunchbase.com/organization/boston-scientific Crunchbase] | [https://www.crunchbase.com/organization/boston-scientific Crunchbase] | ||
[https://www.linkedin.com/company/boston-scientific/ LinkedIn] | [https://www.linkedin.com/company/boston-scientific/ LinkedIn] | ||
[https://github.com/BostonScientific GitHub] | [https://github.com/BostonScientific GitHub] | ||
| + | [https://twitter.com/bostonsci Twitter][https://facebook.com/BostonScientific Facebook] | ||
Latest revision as of 03:03, 18 April 2023
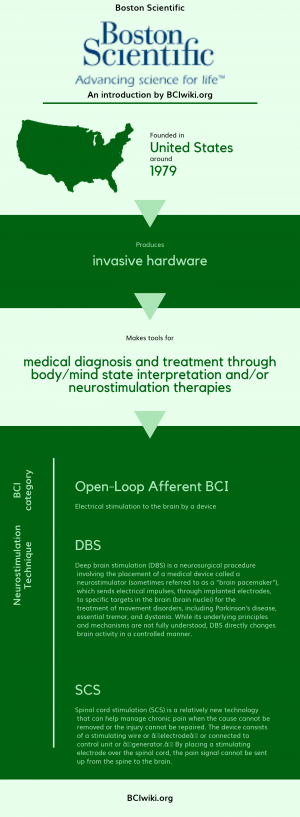
Boston Scientific is a leading innovator of medical solutions that improve the health of patients around the world. Boston Scientific's products and technologies are used to diagnose or treat a wide range of medical conditions, including heart, digestive, pulmonary, vascular, urological, women's health and chronic pain conditions.
Founded in The United States around 1979, Boston Scientific produces invasive hardware.
Boston Scientific makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): DBS, SCS
FDA
Boston Scientific has 3428 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| P130030 | Stent, Coronary | 3 | Cardiovascular | True | False |
| P900056 | Catheter, Coronary, Atherectomy | 3 | Cardiovascular | True | False |
| P920047 | Cardiac Ablation Percutaneous Catheter | 3 | Cardiovascular | True | False |
| P010012 | Defibrillator, Automatic Implantable Cardioverter, With Cardiac Resynchronization (Crt-D) | 3 | Cardiovascular | True | False |
| P920023 | Stent, Urethral, Bulbous, Permanent Or Semi-Permanent | 3 | Gastroenterology, Urology | True | False |
| K163174 | Catheters, Transluminal Coronary Angioplasty, Percutaneous | 2 | Cardiovascular | False | True |
| K191446 | Stent, Ureteral | 2 | Gastroenterology, Urology | False | True |
| K203322 | Dislodger, Stone, Biliary | 2 | Gastroenterology, Urology | False | True |
| K170815 | Analyzer, Pacemaker Generator Function | 2 | Cardiovascular | False | True |
| K133700 | Stents, Drains And Dilators For The Biliary Ducts | 2 | Gastroenterology, Urology | False | True |