If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Cerebra"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 16: | Line 16: | ||
[[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG | ||
| + | ==FDA== | ||
| + | Cerebra has 1 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Cerebra_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K213007 K213007] || Standard Polysomnograph With Electroencephalograph || 2 || Neurology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://cerebrahealth.com/ Website] | [https://cerebrahealth.com/ Website] | ||
[https://www.crunchbase.com/organization/cerebra-health Crunchbase] | [https://www.crunchbase.com/organization/cerebra-health Crunchbase] | ||
[https://www.linkedin.com/company/cerebrahealth/ LinkedIn] | [https://www.linkedin.com/company/cerebrahealth/ LinkedIn] | ||
[https://twitter.com/cerebraofficial Twitter][https://facebook.com/cerebraofficial Facebook][https://instagram.com/cerebraofficial/ Instagram] | [https://twitter.com/cerebraofficial Twitter][https://facebook.com/cerebraofficial Facebook][https://instagram.com/cerebraofficial/ Instagram] | ||
Revision as of 03:08, 18 April 2023
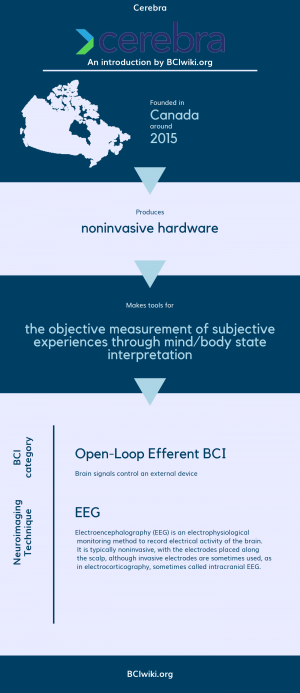
Cerebra was founded with a single purpose: to help people achieve their best sleep possible by understanding sleep where it happens, in the brain.
Founded in Canada around 2015, Cerebra produces noninvasive hardware.
Cerebra makes tools for the objective measurement of subjective experiences through mind/body state interpretation.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EEG
FDA
Cerebra has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K213007 | Standard Polysomnograph With Electroencephalograph | 2 | Neurology | False | True |