If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Nevro Corp"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 15: | Line 15: | ||
[[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: SCS | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: SCS | ||
| + | ==FDA== | ||
| + | Nevro Corp has 44 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Nevro_Corp_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P130022 P130022] || Stimulator, Spinal-Cord, Totally Implanted For Pain Relief || 3 || Neurology || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P130022 P130022] || Stimulator, Spinal-Cord, Totally Implanted For Pain Relief || 3 || Neurology || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P130022 P130022] || Stimulator, Spinal-Cord, Totally Implanted For Pain Relief || 3 || Neurology || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P130022 P130022] || Stimulator, Spinal-Cord, Totally Implanted For Pain Relief || 3 || Neurology || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P130022 P130022] || Stimulator, Spinal-Cord, Totally Implanted For Pain Relief || 3 || Neurology || True || False | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://nevro.com/ Website] | [https://nevro.com/ Website] | ||
[https://www.crunchbase.com/organization/nevro Crunchbase] | [https://www.crunchbase.com/organization/nevro Crunchbase] | ||
[https://www.linkedin.com/company/nevro-hfx/ LinkedIn] | [https://www.linkedin.com/company/nevro-hfx/ LinkedIn] | ||
[https://twitter.com/Nevro_HFX Twitter][https://facebook.com/NevroHFX Facebook] | [https://twitter.com/Nevro_HFX Twitter][https://facebook.com/NevroHFX Facebook] | ||
Revision as of 03:32, 18 April 2023
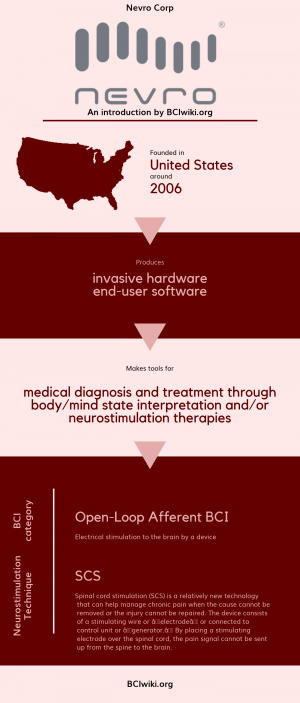
"Nevro is a medical device company headquartered in Redwood City, California that has the simple goal of helping more patients suffering from chronic pain achieve lasting relief."
Founded in The United States around 2006, Nevro Corp produces invasive hardware and end-user software.
Nevro Corp makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): SCS
FDA
Nevro Corp has 44 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| P130022 | Stimulator, Spinal-Cord, Totally Implanted For Pain Relief | 3 | Neurology | True | False |
| P130022 | Stimulator, Spinal-Cord, Totally Implanted For Pain Relief | 3 | Neurology | True | False |
| P130022 | Stimulator, Spinal-Cord, Totally Implanted For Pain Relief | 3 | Neurology | True | False |
| P130022 | Stimulator, Spinal-Cord, Totally Implanted For Pain Relief | 3 | Neurology | True | False |
| P130022 | Stimulator, Spinal-Cord, Totally Implanted For Pain Relief | 3 | Neurology | True | False |