If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "IMediSync"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 16: | Line 16: | ||
[[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG | ||
| + | ==FDA== | ||
| + | iMediSync has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[iMediSync_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K222838 K222838] || Normalizing Quantitative Electroencephalograph Software || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K220056 K220056] || Full-Montage Standard Electroencephalograph || 2 || Neurology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.imedisync.com/en/ Website] | [https://www.imedisync.com/en/ Website] | ||
[https://www.crunchbase.com/organization/imedisync Crunchbase] | [https://www.crunchbase.com/organization/imedisync Crunchbase] | ||
Revision as of 04:12, 18 April 2023
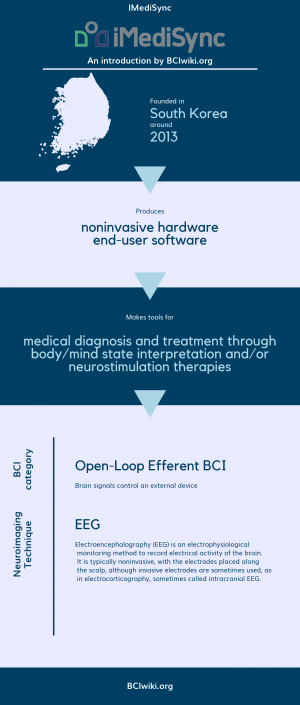
"iMediSync developed the first-ever sex classified EEG/HRV database and AI EEG/HRV automatic analysis cloud platform. The cloud platform iSyncBrain consists of AI automated denoising, a feature extraction pipeline from sensor level to source level, automatic quick summary reports or full reports with the database comparison, and group statistics functionality with a normative library. We completed the KFDA clinical study for our EEG screening biomarker on aMCI detection and have integrated it with the report, iSyncBrain-M1. Various collaborative research is actively undergoing to develop an EEG/HRV based biomarker for Amyloidopathy, coma prognosis, stroke rehabilitation prognosis, Parkinson pathology, depression phenotype, and determining brain lobe age for children. This will be known as the iSyncBrain-M series."
Founded in South Korea around 2013, IMediSync produces noninvasive hardware and end-user software.
IMediSync makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EEG
FDA
iMediSync has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K222838 | Normalizing Quantitative Electroencephalograph Software | 2 | Neurology | False | True |
| K220056 | Full-Montage Standard Electroencephalograph | 2 | Neurology | False | True |
Links
Website Crunchbase LinkedIn LinkedIn 2 Twitter YouTube Instagram