If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Cerebra"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 23: | Line 23: | ||
! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K213007 K213007] || Standard Polysomnograph With Electroencephalograph || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K213007 K213007] || Standard Polysomnograph With Electroencephalograph || 2 || Neurology || False || True |
|} | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
Latest revision as of 22:56, 17 February 2024
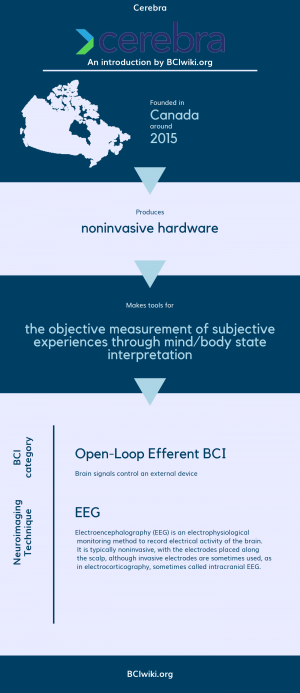
Cerebra was founded with a single purpose: to help people achieve their best sleep possible by understanding sleep where it happens, in the brain.
Founded in Canada around 2015, Cerebra produces noninvasive hardware.
Cerebra makes tools for the objective measurement of subjective experiences through mind/body state interpretation.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EEG
FDA
Cerebra has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K213007 | Standard Polysomnograph With Electroencephalograph | 2 | Neurology | False | True |