If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "CorTec"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Category:LinkedIn Accounts]] | ||
| + | [[Category:Facebook Pages]] | ||
| + | [[Category:Twitter Accounts]] | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| Line 7: | Line 10: | ||
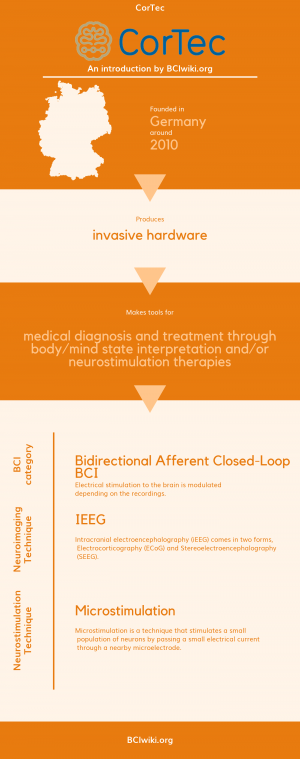
Founded in Germany around 2010, CorTec produces invasive hardware. | Founded in Germany around 2010, CorTec produces invasive hardware. | ||
| − | CorTec makes tools for medical diagnosis and treatment through body/mind state interpretation and neurostimulation therapies. | + | CorTec makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies. |
[[Brain Computer Interface Classification|BCI Categories]]: Bidirectional Afferent Closed-Loop | [[Brain Computer Interface Classification|BCI Categories]]: Bidirectional Afferent Closed-Loop | ||
| + | |||
| + | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: iEEG | ||
| + | |||
| + | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: Microstimulation | ||
| + | ==FDA== | ||
| + | CorTec has 1 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[CorTec_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K183437 K183437] || Electrode, Cortical || 2 || Neurology || False || True | ||
| + | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
| + | |||
[https://www.cortec-neuro.com/ Website] | [https://www.cortec-neuro.com/ Website] | ||
[https://www.crunchbase.com/organization/cortec Crunchbase] | [https://www.crunchbase.com/organization/cortec Crunchbase] | ||
[https://www.linkedin.com/company/cortec-gmbh/ LinkedIn] | [https://www.linkedin.com/company/cortec-gmbh/ LinkedIn] | ||
| + | [https://twitter.com/_CorTec_ Twitter][https://facebook.com/CorTecNeuro/?fref=ts Facebook] | ||
Latest revision as of 22:56, 17 February 2024
"CorTec is a young company in the field of medical engineering. We are developing the world's most efficient implants serving as a direct interface between brain and technology. CorTec Brain Interchange is an new brain pacemaker technology that we are offering to our customers for the treatment of neurological diseases. The completely implantable system is able to record brain activity and influence it by electrical stimulation. The unique feature of CorTec Brain Interchange is that it is able to control itself (closed-loop technology): The system records the brain's activity and thus the effect of its stimulation. It then aligns its further activities with the recorded data. Neurotechnological implants currently available on the market work via externally controlled continuous stimulation of the brain. CorTec Brain Interchange determines the respective therapeutic demand in direct communication with the brain and thus allows for more gentle treatment."
Founded in Germany around 2010, CorTec produces invasive hardware.
CorTec makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Bidirectional Afferent Closed-Loop
Neurosensing Technique(s): iEEG
Neurostimulation Technique(s): Microstimulation
FDA
CorTec has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K183437 | Electrode, Cortical | 2 | Neurology | False | True |