If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Infinite Biomedical Technologies"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| − | Infinite Biomedical Technologies, LLC is a translational research and medical device company which takes technology from bench to bedside. Our current mission is to develop innovative upper limb prosthetic devices and control systems that functionally improve the lives of amputees. Specifically, we are developing technology at the interface between people and their prosthetics. | + | "Infinite Biomedical Technologies, LLC is a translational research and medical device company which takes technology from bench to bedside. Our current mission is to develop innovative upper limb prosthetic devices and control systems that functionally improve the lives of amputees. Specifically, we are developing technology at the interface between people and their prosthetics." |
| Line 16: | Line 16: | ||
[[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EMG | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EMG | ||
| + | |||
| + | ==Software== | ||
| + | *[[Sense|Sense]] | ||
| + | ==FDA== | ||
| + | Infinite Biomedical Technologies has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Infinite_Biomedical_Technologies_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K173571 K173571] || Electrode, Cutaneous || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K182112 K182112] || Electrode, Cutaneous || 2 || Neurology || False || True | ||
| + | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
| + | |||
[https://www.i-biomed.com/ Website] | [https://www.i-biomed.com/ Website] | ||
[https://www.crunchbase.com/organization/infinite-biomedical-technologies Crunchbase] | [https://www.crunchbase.com/organization/infinite-biomedical-technologies Crunchbase] | ||
Latest revision as of 22:57, 17 February 2024
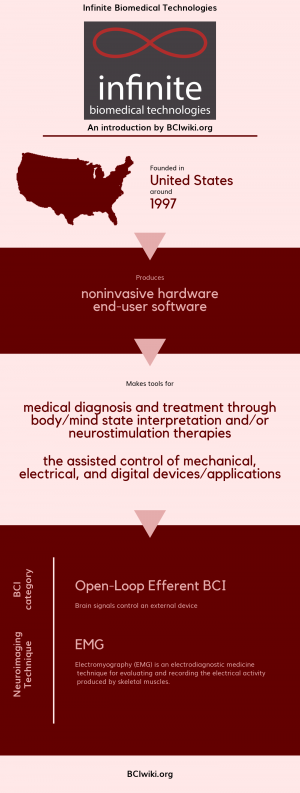
"Infinite Biomedical Technologies, LLC is a translational research and medical device company which takes technology from bench to bedside. Our current mission is to develop innovative upper limb prosthetic devices and control systems that functionally improve the lives of amputees. Specifically, we are developing technology at the interface between people and their prosthetics."
Founded in The United States around 1997, Infinite Biomedical Technologies produces noninvasive hardware and end-user software.
Infinite Biomedical Technologies makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies and the assisted control of mechanical,electrical,and digital devices/applications.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EMG
Software
FDA
Infinite Biomedical Technologies has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K173571 | Electrode, Cutaneous | 2 | Neurology | False | True |
| K182112 | Electrode, Cutaneous | 2 | Neurology | False | True |