If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "MicroTransponder"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 14: | Line 14: | ||
[[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: VNS | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: VNS | ||
| + | ==FDA== | ||
| + | MicroTransponder has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[MicroTransponder_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P210007 P210007] || Stimulator, Autonomic Nerve, Implanted For Stroke Rehabilitation || 3 || Physical Medicine || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P210007 P210007] || Stimulator, Autonomic Nerve, Implanted For Stroke Rehabilitation || 3 || Physical Medicine || True || False | ||
| + | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
| + | |||
[https://microtransponder.com/en Website] | [https://microtransponder.com/en Website] | ||
[https://www.crunchbase.com/organization/microtransponder Crunchbase] | [https://www.crunchbase.com/organization/microtransponder Crunchbase] | ||
[https://www.linkedin.com/company/microtransponder/ LinkedIn] | [https://www.linkedin.com/company/microtransponder/ LinkedIn] | ||
[https://twitter.com/Microtranspondr Twitter] | [https://twitter.com/Microtranspondr Twitter] | ||
Latest revision as of 22:57, 17 February 2024
"MicroTransponder has developed the Paired Vagus Nerve Stimulation System (Paired VNS System) based on decades of neuroscience research. The Paired VNS System is designed to treat several neurological conditions. Our initial focus is upper limb deficits among chronic stroke patients. The Vivistim System enables stroke survivors to regain upper limb mobility."
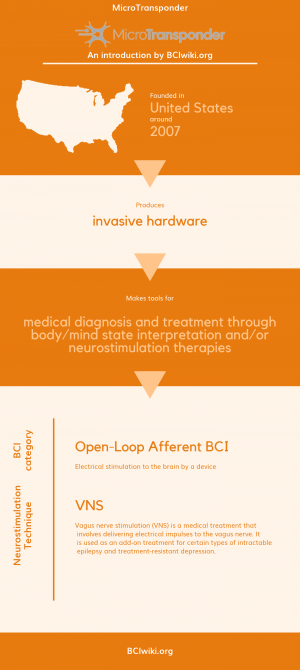
Founded in The United States around 2007, MicroTransponder produces invasive hardware.
MicroTransponder makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): VNS
FDA
MicroTransponder has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| P210007 | Stimulator, Autonomic Nerve, Implanted For Stroke Rehabilitation | 3 | Physical Medicine | True | False |
| P210007 | Stimulator, Autonomic Nerve, Implanted For Stroke Rehabilitation | 3 | Physical Medicine | True | False |