If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Neurolief"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 14: | Line 14: | ||
[[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: TENS | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: TENS | ||
| + | ==FDA== | ||
| + | Neurolief has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Neurolief_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K203419 K203419] || Stimulator, Nerve, Electrical, Transcutaneous, For Migraine || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K212106 K212106] || Stimulator, Nerve, Electrical, Transcutaneous, For Migraine || 2 || Neurology || False || True | ||
| + | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
| + | |||
[https://www.neurolief.com/ Website] | [https://www.neurolief.com/ Website] | ||
[https://www.crunchbase.com/organization/neurolief Crunchbase] | [https://www.crunchbase.com/organization/neurolief Crunchbase] | ||
[https://www.linkedin.com/company/neurolief/ LinkedIn] | [https://www.linkedin.com/company/neurolief/ LinkedIn] | ||
[https://twitter.com/NeuroliefInc Twitter] | [https://twitter.com/NeuroliefInc Twitter] | ||
Latest revision as of 22:57, 17 February 2024
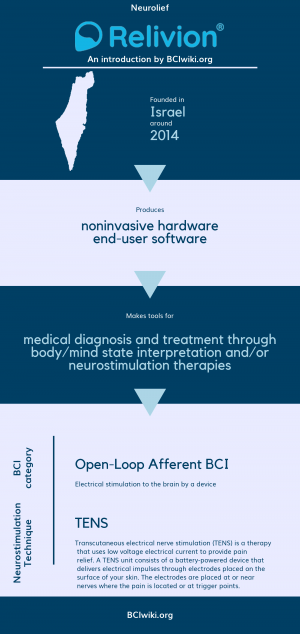
"Neuromodulation is clinically proven to alter brain function through targeted, delivery of currents to the central nervous system. Relivionr is the first non-invasive multi-channel brain neuromodulation technology that maximizes this therapeutic effect by personalizing treatment via precise, selective activation of major neural pathways. Effective treatment is realized without the risks and costs associated with invasive procedures and without the side effects associated with medications."
Founded in Israel around 2014, Neurolief produces noninvasive hardware and end-user software.
Neurolief makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): TENS
FDA
Neurolief has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K203419 | Stimulator, Nerve, Electrical, Transcutaneous, For Migraine | 2 | Neurology | False | True |
| K212106 | Stimulator, Nerve, Electrical, Transcutaneous, For Migraine | 2 | Neurology | False | True |