If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Stimwave Technologies"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 23: | Line 23: | ||
! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K191466 K191466] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K191466 K191466] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True |
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K170141 K170141] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K170141 K170141] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True |
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K162161 K162161] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K162161 K162161] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True |
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K182720 K182720] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K182720 K182720] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True |
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K172644 K172644] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K172644 K172644] || Stimulator, Spinal-Cord, Implanted (Pain Relief) || 2 || Neurology || False || True |
|} | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
Latest revision as of 22:58, 17 February 2024
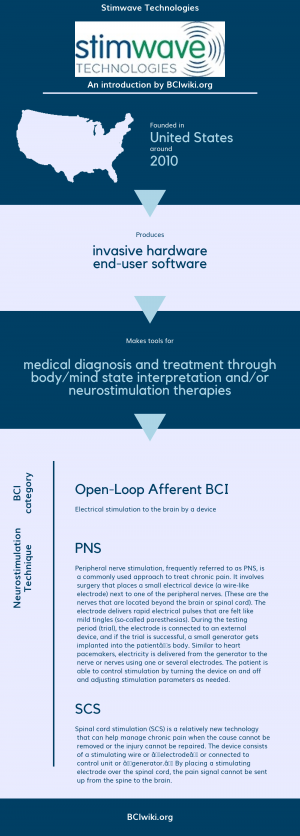
"Stimwave Technologies, Inc., a medical device company, engages in the development, manufacture, commercialization, and marketing of wireless microsize injectable medical devices for neurology markets. The company was formerly known as Neural Micro Incorporated. Stimwave Technologies, Inc. was incorporated in 2010 and is based in Scottsdale, Arizona."
Founded in The United States around 2010, Stimwave Technologies produces invasive hardware and end-user software.
Stimwave Technologies makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): SCS
FDA
Stimwave Technologies has 7 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K191466 | Stimulator, Spinal-Cord, Implanted (Pain Relief) | 2 | Neurology | False | True |
| K170141 | Stimulator, Spinal-Cord, Implanted (Pain Relief) | 2 | Neurology | False | True |
| K162161 | Stimulator, Spinal-Cord, Implanted (Pain Relief) | 2 | Neurology | False | True |
| K182720 | Stimulator, Spinal-Cord, Implanted (Pain Relief) | 2 | Neurology | False | True |
| K172644 | Stimulator, Spinal-Cord, Implanted (Pain Relief) | 2 | Neurology | False | True |