If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "NeuroEM"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Category:LinkedIn Accounts]] | ||
| + | [[Category:Instagram Pages]] | ||
| + | [[Category:Facebook Pages]] | ||
| + | [[Category:Twitter Accounts]] | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| − | NeuroEM Therapeutics has developed a first-in-class, self-contained head device (the MemorEMT) to treat AD with electromagnetic waves - a therapy that we have pioneered and tested successfully in AD animal studies with no adverse events seen. Our novel, proprietary technology appears to directly affect the AD process to prevent and reverse memory impairment. The MemorEM head device, which allows for complete mobility during one-hour treatments "in home", has been successfully used in NeuroEM | + | "NeuroEM Therapeutics has developed a first-in-class, self-contained head device (the MemorEMT) to treat AD with electromagnetic waves - a therapy that we have pioneered and tested successfully in AD animal studies with no adverse events seen. Our novel, proprietary technology appears to directly affect the AD process to prevent and reverse memory impairment. The MemorEM head device, which allows for complete mobility during one-hour treatments "in home", has been successfully used in NeuroEM's recently-completed Pilot clinical trial that evaluated the safety and efficacy of Transcranial Electromagnetic Treatment (TEMT) in Alzheimer's patients. In view of its promising effects on memory in this clinical trial, TEMT could be an entirely new bioengineering-based intervention against AD." |
| Line 15: | Line 19: | ||
[https://neuroem.com/ Website] | [https://neuroem.com/ Website] | ||
[https://www.linkedin.com/company/neuroem-therapeutics-inc/ LinkedIn] | [https://www.linkedin.com/company/neuroem-therapeutics-inc/ LinkedIn] | ||
| + | [https://twitter.com/NeuroEMTherapy Twitter][https://facebook.com/neuroem/ Facebook][https://instagram.com/neuroemtherapy Instagram] | ||
Latest revision as of 00:16, 9 August 2022
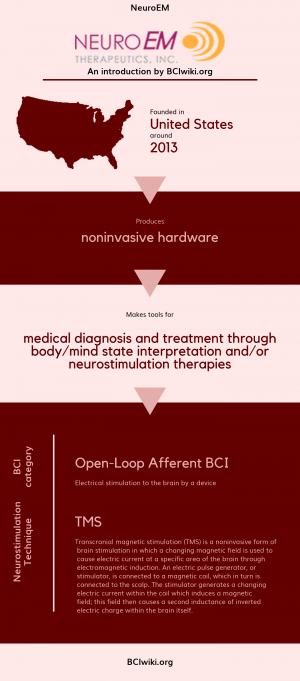
"NeuroEM Therapeutics has developed a first-in-class, self-contained head device (the MemorEMT) to treat AD with electromagnetic waves - a therapy that we have pioneered and tested successfully in AD animal studies with no adverse events seen. Our novel, proprietary technology appears to directly affect the AD process to prevent and reverse memory impairment. The MemorEM head device, which allows for complete mobility during one-hour treatments "in home", has been successfully used in NeuroEM's recently-completed Pilot clinical trial that evaluated the safety and efficacy of Transcranial Electromagnetic Treatment (TEMT) in Alzheimer's patients. In view of its promising effects on memory in this clinical trial, TEMT could be an entirely new bioengineering-based intervention against AD."
Founded in The United States around 2013, NeuroEM produces noninvasive hardware.
NeuroEM makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): TMS