If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Nexstim"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Category:YouTube Channels]] | ||
| + | [[Category:Twitter Accounts]] | ||
| + | [[Category:LinkedIn Accounts]] | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| − | Nexstim Oy is a medical device company developing Navigated Brain Stimulation (NBS) - noninvasive, image-guided transcranial magnetic stimulation (TMS) - for brain diagnostics and therapy. The Nexstim NBS System is rapidly becoming the new standard for preoperative functional brain mapping prior to challenging neurosurgery for tumor or epilepsy. The accuracy of the NBS System has been shown to be equivalent to that of invasive direct cortical stimulation during actual brain tumor surgery, hitherto considered the "gold standard" method for locating the motor cortex. | + | "Nexstim Oy is a medical device company developing Navigated Brain Stimulation (NBS) - noninvasive, image-guided transcranial magnetic stimulation (TMS) - for brain diagnostics and therapy. The Nexstim NBS System is rapidly becoming the new standard for preoperative functional brain mapping prior to challenging neurosurgery for tumor or epilepsy. The accuracy of the NBS System has been shown to be equivalent to that of invasive direct cortical stimulation during actual brain tumor surgery, hitherto considered the "gold standard" method for locating the motor cortex." |
| + | |||
| + | |||
| + | [[File:Nexstim.png|thumb|Nexstim Company Profile]] | ||
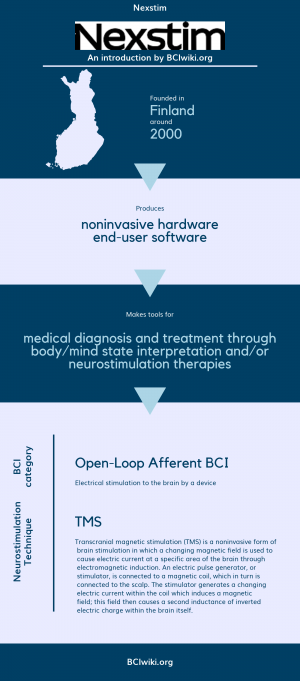
| + | Founded in Finland around 2000, Nexstim produces noninvasive hardware and end-user software. | ||
| + | |||
| + | Nexstim makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies. | ||
| + | |||
| + | [[Brain Computer Interface Classification|BCI Categories]]: Open-Loop Afferent | ||
| + | |||
| + | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: TMS | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[NBS_5_Motor_Mapping_System|NBS 5 Motor Mapping System]] | ||
| + | *[[NBT_System_2|NBT System 2]] | ||
| + | ==FDA== | ||
| + | Nexstim has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Nexstim_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K171902 K171902] || Transcranial Magnetic Stimulator || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K182700 K182700] || Transcranial Magnetic Stimulator || 2 || Neurology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[http://www.nexstim.com Website] | [http://www.nexstim.com Website] | ||
[https://www.crunchbase.com/organization/nexstim Crunchbase] | [https://www.crunchbase.com/organization/nexstim Crunchbase] | ||
[https://www.linkedin.com/company/nexstim/ LinkedIn] | [https://www.linkedin.com/company/nexstim/ LinkedIn] | ||
| + | [https://twitter.com/NexstimOyj Twitter] | ||
| + | [https://youtube.com/user/Nexstim YouTube] | ||
Latest revision as of 02:26, 18 April 2023
"Nexstim Oy is a medical device company developing Navigated Brain Stimulation (NBS) - noninvasive, image-guided transcranial magnetic stimulation (TMS) - for brain diagnostics and therapy. The Nexstim NBS System is rapidly becoming the new standard for preoperative functional brain mapping prior to challenging neurosurgery for tumor or epilepsy. The accuracy of the NBS System has been shown to be equivalent to that of invasive direct cortical stimulation during actual brain tumor surgery, hitherto considered the "gold standard" method for locating the motor cortex."
Founded in Finland around 2000, Nexstim produces noninvasive hardware and end-user software.
Nexstim makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): TMS
Hardware
FDA
Nexstim has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K171902 | Transcranial Magnetic Stimulator | 2 | Neurology | False | True |
| K182700 | Transcranial Magnetic Stimulator | 2 | Neurology | False | True |