If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Advanced Brain Monitoring"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Category:LinkedIn Accounts]] | ||
| + | [[Category:Twitter Accounts]] | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| Line 19: | Line 21: | ||
==Developer Tools== | ==Developer Tools== | ||
*[[B-Alert_Live|B-Alert Live]] | *[[B-Alert_Live|B-Alert Live]] | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[Sleep_Profiler|Sleep Profiler]] | ||
| + | *[[Sleep_Profiler_PSG2|Sleep Profiler PSG2]] | ||
| + | *[[Stat_X-Series_mobile_EEG|Stat X-Series mobile EEG]] | ||
| + | ==FDA== | ||
| + | Advanced Brain Monitoring has 1 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Advanced_Brain_Monitoring_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K172799 K172799] || Ventilatory Effort Recorder || 2 || Anesthesiology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.advancedbrainmonitoring.com/ Website] | [https://www.advancedbrainmonitoring.com/ Website] | ||
[https://www.crunchbase.com/organization/advanced-brain-monitoring-inc Crunchbase] | [https://www.crunchbase.com/organization/advanced-brain-monitoring-inc Crunchbase] | ||
[https://www.linkedin.com/company/advanced-brain-monitoring/ LinkedIn] | [https://www.linkedin.com/company/advanced-brain-monitoring/ LinkedIn] | ||
| + | [https://twitter.com/balert Twitter] | ||
Latest revision as of 23:40, 2 February 2024
"Over the past decade, Advanced Brain Monitoring has developed and implemented mobile, user-friendly platforms for acquiring, integrating, analyzing and reporting multi-sensor data in real-world applications. Our strong and growing technical team includes PhD- and masters-level hardware engineers, biostatisticians, software engineers and experts in signal processing, experimental design and clinical trials. ABM seeks to build on existing collaborations within the academic community and develop strategic partnerships across industry with like-minded professionals."
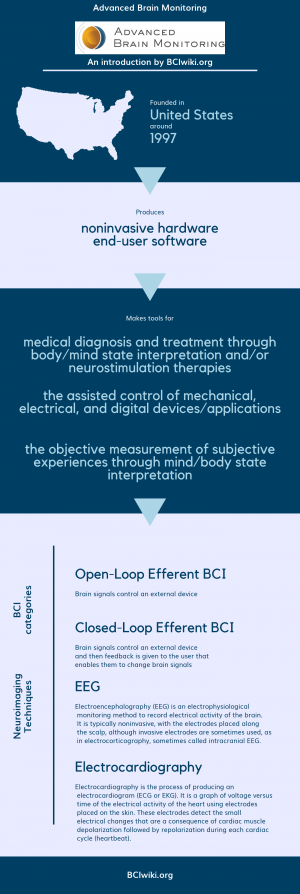
Founded in The United States around 1997, Advanced Brain Monitoring produces noninvasive hardware and end-user software.
Advanced Brain Monitoring makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies, the assisted control of mechanical,electrical,and digital devices/applications and the objective measurement of subjective experiences through mind/body state interpretation.
BCI Categories: Open-Loop Efferent, Closed-Loop Efferent
Neurosensing Technique(s): EEG
Software
Developer Tools
Hardware
FDA
Advanced Brain Monitoring has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K172799 | Ventilatory Effort Recorder | 2 | Anesthesiology | False | True |