If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Omniscient Neurotechnology"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 16: | Line 16: | ||
[[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: MRI, FMRI | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: MRI, FMRI | ||
| + | ==FDA== | ||
| + | Omniscient Neurotechnology has 1 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Omniscient_Neurotechnology_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K203518 K203518] || System, Image Processing, Radiological || 2 || Radiology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.o8t.com/ Website] | [https://www.o8t.com/ Website] | ||
[https://www.crunchbase.com/organization/omniscient-neurotechnology Crunchbase] | [https://www.crunchbase.com/organization/omniscient-neurotechnology Crunchbase] | ||
[https://www.linkedin.com/company/omniscientneurotechnology/ LinkedIn] | [https://www.linkedin.com/company/omniscientneurotechnology/ LinkedIn] | ||
[https://twitter.com/o8tneuro Twitter] | [https://twitter.com/o8tneuro Twitter] | ||
Revision as of 04:13, 18 April 2023
"We create enterprise grade clinical and research solutions applicable to a variety of brain-related disorders, including depression, chronic pain, cancer, bi-polar disorder, Alzheimer’s, dementia, and PTSD, with the goal of bringing predictive analytics to the world faster.
We are a data company and aim to radically change the care of patients with brain disease by turning loose Machine learning/AI and big data to actually provide useful information to doctors. This involves both facilitating research, and providing high quality data based guidance to clinicians."
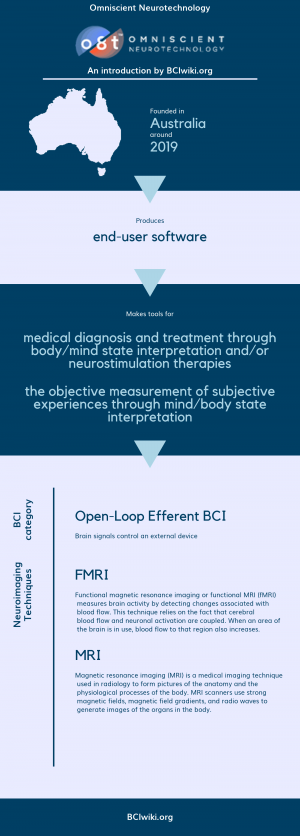
Founded in Australia around 2019, Omniscient Neurotechnology produces end-user software.
Omniscient Neurotechnology makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies and the objective measurement of subjective experiences through mind/body state interpretation.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): MRI, FMRI
FDA
Omniscient Neurotechnology has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K203518 | System, Image Processing, Radiological | 2 | Radiology | False | True |