If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "OpenFDA Integration"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) (Created page with "OpenFDA_Integration "openFDA provides APIs and raw download access to a number of high-value, high priority and scalable structured datasets, including adverse events, drug p...") |
Landonodnal (talk | contribs) |
||
| Line 7: | Line 7: | ||
An explanation of the FDA approval process for medical devices can be found [https://www.sciencedirect.com/science/article/pii/S2452302X16300183 here]. | An explanation of the FDA approval process for medical devices can be found [https://www.sciencedirect.com/science/article/pii/S2452302X16300183 here]. | ||
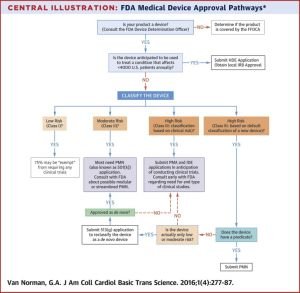
| − | [[File:fda-medical-device-approval-pathways.jpg|thumb| Flowchart of FDA medical device approval pathways | + | [[File:fda-medical-device-approval-pathways.jpg|thumb| Flowchart of FDA medical device approval pathways |
| + | |||
| + | Source: https://www.sciencedirect.com/science/article/pii/S2452302X16300183]] | ||
Revision as of 18:03, 15 April 2023
OpenFDA_Integration
"openFDA provides APIs and raw download access to a number of high-value, high priority and scalable structured datasets, including adverse events, drug product labeling, and recall enforcement reports." Find out more here.
The FDA also offers Devices@FDA for searching medical device databases.
An explanation of the FDA approval process for medical devices can be found here.

Flowchart of FDA medical device approval pathways Source: https://www.sciencedirect.com/science/article/pii/S2452302X16300183