If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "OpenFDA Integration"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
"openFDA provides APIs and raw download access to a number of high-value, high priority and scalable structured datasets, including adverse events, drug product labeling, and recall enforcement reports." Find out more [https://open.fda.gov/ here]. | "openFDA provides APIs and raw download access to a number of high-value, high priority and scalable structured datasets, including adverse events, drug product labeling, and recall enforcement reports." Find out more [https://open.fda.gov/ here]. | ||
Revision as of 18:04, 15 April 2023
"openFDA provides APIs and raw download access to a number of high-value, high priority and scalable structured datasets, including adverse events, drug product labeling, and recall enforcement reports." Find out more here.
The FDA also offers Devices@FDA for searching medical device databases.
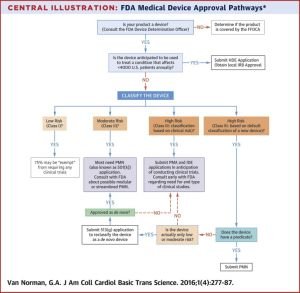
An explanation of the FDA approval process for medical devices can be found here.

Flowchart of FDA medical device approval pathways - Source: https://www.sciencedirect.com/science/article/pii/S2452302X16300183