If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "NeuroSigma"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 20: | Line 20: | ||
! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=DEN180041 DEN180041] || Transcutaneous Nerve Stimulator For Adhd || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=DEN180041 DEN180041] || Transcutaneous Nerve Stimulator For Adhd || 2 || Neurology || False || True |
|} | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
Latest revision as of 22:57, 17 February 2024
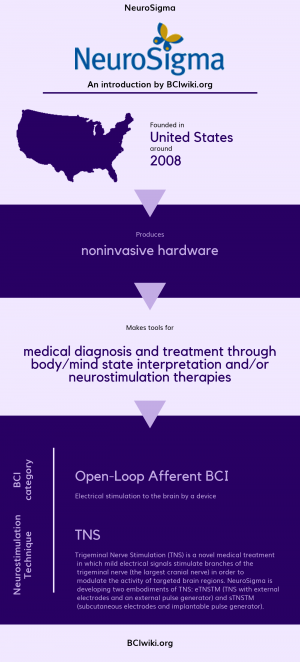
"NeuroSigma is a California-based life sciences company established to develop bioelectronic technologies with the potential to transform medical practice and patients' lives. NeuroSigma is focused on TNS neuromodulation therapies based on intellectual property, licensed on an exclusive basis from the University of California, Los Angeles (UCLA), covering a wide-spectrum of disorders, including ADHD."
Founded in The United States around 2008, NeuroSigma produces noninvasive hardware.
NeuroSigma makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): TNS
FDA
NeuroSigma has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| DEN180041 | Transcutaneous Nerve Stimulator For Adhd | 2 | Neurology | False | True |