If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Lifetech"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 13: | Line 13: | ||
[[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG, NIRS | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG, NIRS | ||
| + | ==FDA== | ||
| + | Lifetech has 1 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Lifetech_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K182839 K182839] || Pulse-Generator, Pacemaker, External || 2 || Cardiovascular || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[http://www.lifetechinc.co.kr/ Website] | [http://www.lifetechinc.co.kr/ Website] | ||
Revision as of 03:21, 18 April 2023
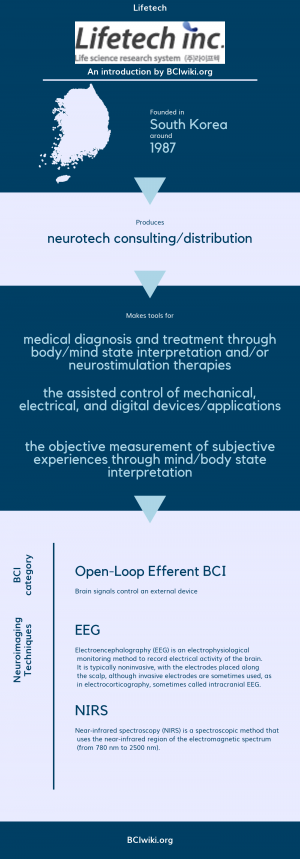
"Lifetech Co., Ltd. has been making continuous efforts for the development of domestic biotechnology since its establishment in 1987. Lifetech, which has developed with the interest and love of customers, now wants to reward customers with upgraded services. We will do our best to develop optimal solutions to keep pace with the rapidly changing research environment. thank you."
Founded in South Korea around 1987, Lifetech produces neurotech consulting services.
Lifetech makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies, the assisted control of mechanical,electrical,and digital devices/applications and the objective measurement of subjective experiences through mind/body state interpretation.
BCI Categories: Open-Loop Efferent,
Neurosensing Technique(s): EEG, NIRS
FDA
Lifetech has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K182839 | Pulse-Generator, Pacemaker, External | 2 | Cardiovascular | False | True |