If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Inomed"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 16: | Line 16: | ||
[[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: tDCS, TMS | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: tDCS, TMS | ||
| + | ==FDA== | ||
| + | Inomed has 1 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Inomed_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K223254 K223254] || Stimulator, Nerve || 2 || Ear, Nose, Throat || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.inomed.com/ Website] | [https://www.inomed.com/ Website] | ||
[https://www.linkedin.com/company/inomed/ LinkedIn] | [https://www.linkedin.com/company/inomed/ LinkedIn] | ||
[https://twitter.com/InomedNeurocare Twitter] | [https://twitter.com/InomedNeurocare Twitter] | ||
Revision as of 01:58, 18 April 2023
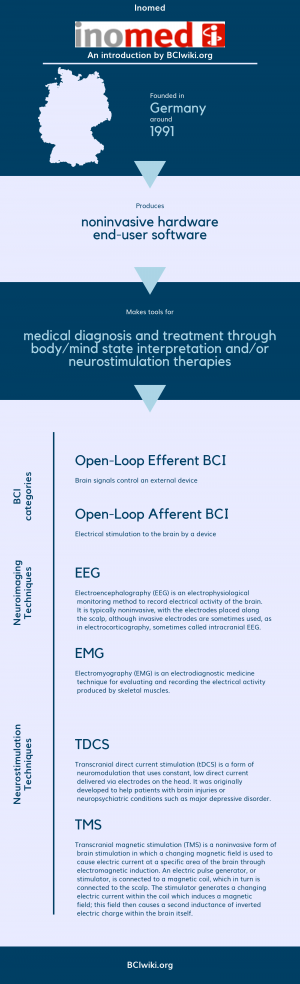
'inomed is a leading medical device company. We develop products that help clinicians satisfy the basic principal of the Hippocratic Oath, "Primum nil nocere" - "First, do no harm". Within the context of this solemn undertaking, patient safety is the highest priority for every clinician. And while patient safety and welfare are priorities within all aspects of medicine, patient safety takes a preeminent role in the more complex and sophisticated neuro-diagnostics, neurosurgical interventions and related therapies.'
Founded in Germany around 1991, Inomed produces noninvasive hardware and end-user software.
Inomed makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent, Open-Loop Afferent
Neurosensing Technique(s): EEG, EMG
Neurostimulation Technique(s): tDCS, TMS
FDA
Inomed has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K223254 | Stimulator, Nerve | 2 | Ear, Nose, Throat | False | True |