If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "BrainsWay"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 14: | Line 14: | ||
[[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: TMS | [[:Category:Neurostimulation_Techniques|Neurostimulation Technique(s)]]: TMS | ||
| + | ==FDA== | ||
| + | BrainsWay has 1 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[BrainsWay_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K220819 K220819] || Transcranial Magnetic Stimulator || 2 || Neurology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.brainsway.com/ Website] | [https://www.brainsway.com/ Website] | ||
[https://www.crunchbase.com/organization/brainsway Crunchbase] | [https://www.crunchbase.com/organization/brainsway Crunchbase] | ||
[https://www.linkedin.com/company/brainsway/ LinkedIn] | [https://www.linkedin.com/company/brainsway/ LinkedIn] | ||
[https://twitter.com/BrainsWay Twitter] | [https://twitter.com/BrainsWay Twitter] | ||
Revision as of 03:07, 18 April 2023
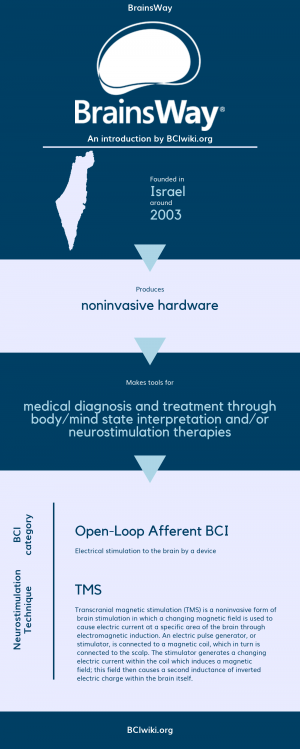
Brainsway's patented breakthrough technology launches a new era in brain disorder treatment.
Founded in Israel around 2003, BrainsWay produces noninvasive hardware.
BrainsWay makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Afferent
Neurostimulation Technique(s): TMS
FDA
BrainsWay has 1 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K220819 | Transcranial Magnetic Stimulator | 2 | Neurology | False | True |