If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Persyst"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| Line 21: | Line 21: | ||
! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K222002 K222002] || Automatic Event Detection Software For Full-Montage Electroencephalograph || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K222002 K222002] || Automatic Event Detection Software For Full-Montage Electroencephalograph || 2 || Neurology || False || True |
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K182181 K182181] || Automatic Event Detection Software For Full-Montage Electroencephalograph || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K182181 K182181] || Automatic Event Detection Software For Full-Montage Electroencephalograph || 2 || Neurology || False || True |
|- | |- | ||
| − | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K171184 K171184] || Automatic Event Detection Software For Full-Montage Electroencephalograph || 2 || Neurology || False || True | + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K171184 K171184] || Automatic Event Detection Software For Full-Montage Electroencephalograph || 2 || Neurology || False || True |
|} | |} | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[BrainScope|BrainScope]] | ||
==Links== | ==Links== | ||
Latest revision as of 22:57, 17 February 2024
"Persyst 14 EEG Review and Analysis Software provides the complete set of tools needed for C.A.R.E (Computer Assisted Review of EEG), resulting in accurate, efficient and rapid review of EEG data. Whether for high volume institutions or for community clinics, Persyst enables the highest level of comprehensive patient care when it comes to EEG monitoring and analysis."
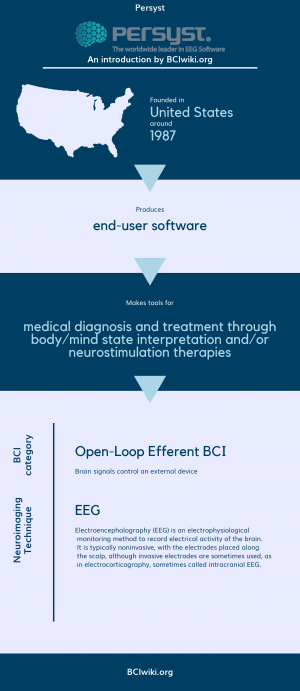
Founded in The United States around 1987, Persyst produces end-user software.
Persyst makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EEG
FDA
Persyst has 3 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K222002 | Automatic Event Detection Software For Full-Montage Electroencephalograph | 2 | Neurology | False | True |
| K182181 | Automatic Event Detection Software For Full-Montage Electroencephalograph | 2 | Neurology | False | True |
| K171184 | Automatic Event Detection Software For Full-Montage Electroencephalograph | 2 | Neurology | False | True |