If you're interested in becoming a contributor or requesting changes then click here to join the discord
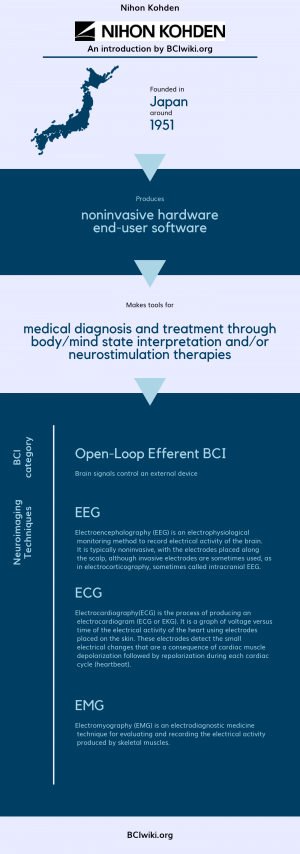
Nihon Kohden
"Nihon Kohden Corporation is a Tokyo-based leading manufacturer, developer and distributor of medical electronic equipment, which include EEGs, EMG measuring systems, ECGs, patient monitors and clinical information systems, with subsidiaries in the U.S., Europe and Asia. The company's products are now used in more than 120 countries, and it is the largest supplier of EEG products worldwide."
Founded in Japan around 1951, Nihon Kohden produces noninvasive hardware and end-user software.
Nihon Kohden makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EEG
Software
FDA
Nihon Kohden has 17 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K220989 | Monitor, Physiological, Patient(With Arrhythmia Detection Or Alarms) | 2 | Cardiovascular | False | True |
| K163459 | Monitor, Physiological, Patient(With Arrhythmia Detection Or Alarms) | 2 | Cardiovascular | False | True |
| K181695 | Ventilator, Continuous, Facility Use | 2 | Anesthesiology | False | True |
| K192307 | Ventilator, Continuous, Facility Use | 2 | Anesthesiology | False | True |
| K213316 | Stimulator, Nerve, Peripheral, Electric | 2 | Anesthesiology | False | True |