If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Compumedics Limited"
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 7: | Line 7: | ||
[[File:Compumedics_Limited.png|thumb|Compumedics Limited Company Profile]] | [[File:Compumedics_Limited.png|thumb|Compumedics Limited Company Profile]] | ||
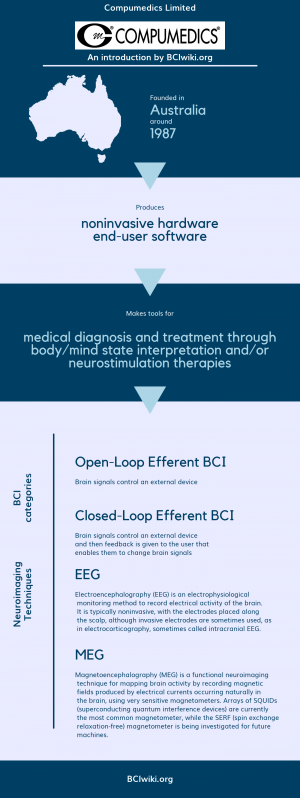
| − | Founded in | + | Founded in Australia around 1987, Compumedics Limited produces noninvasive hardware and end-user software. |
Compumedics Limited makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies. | Compumedics Limited makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies. | ||
| Line 20: | Line 20: | ||
*[[Profusion_Sleep_Software|Profusion Sleep Software]] | *[[Profusion_Sleep_Software|Profusion Sleep Software]] | ||
*[[Somt%C3%A9_Software|Somté Software]] | *[[Somt%C3%A9_Software|Somté Software]] | ||
| + | |||
| + | ==Hardware== | ||
| + | *[[Grael_4K_PSG:EEG|Grael 4K PSG:EEG]] | ||
| + | *[[Grael_4K-EEG|Grael 4K-EEG]] | ||
| + | *[[Grael_LT_EEG_System|Grael LT EEG System]] | ||
| + | *[[Neuvo_64-512_Channel_LTM_EEG|Neuvo 64-512 Channel LTM EEG]] | ||
| + | *[[Okti|Okti]] | ||
| + | *[[ONsight_A.V.S.|ONsight A.V.S.]] | ||
| + | *[[Siesta|Siesta]] | ||
| + | *[[Xegis Forte EMG/EP]] | ||
| + | *[[Xegis G:NEO 2 Channel EMG/EP]] | ||
| + | ==FDA== | ||
| + | Compumedics Limited has 2 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Compumedics_Limited_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K191785 K191785] || Magnetoencephalograph || 2 || Neurology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K230073 K230073] || Full-Montage Standard Electroencephalograph || 2 || Neurology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
| + | |||
[https://www.compumedics.com.au/ Website] | [https://www.compumedics.com.au/ Website] | ||
[https://www.crunchbase.com/organization/compumedics Crunchbase] | [https://www.crunchbase.com/organization/compumedics Crunchbase] | ||
[https://www.linkedin.com/company/compumedics/ LinkedIn] | [https://www.linkedin.com/company/compumedics/ LinkedIn] | ||
[https://twitter.com/Compumedics Twitter] | [https://twitter.com/Compumedics Twitter] | ||
Latest revision as of 01:47, 18 April 2023
"Compumedics Limited (ASX: CMP) is a medical device company involved in the development, manufacture and commercialisation of diagnostics technology for the sleep, brain and ultrasonic blood-flow monitoring applications. The Company owns US based Neuroscan and Germany-based DWL Elektronishe Systeme GmbH. In conjunction with these two subsidiaries, Compumedics has a broad international reach, including Americas; Australia and Asia Pacific; and Europe and the Middle East. Executive Chairman, Dr David Burton, founded Compumedics in 1987. In the same year the Company successfully designed and installed the first Australian, fully computerised Sleep Clinic at Epworth Hospital in Melbourne. Following this early success, Compumedics focused on the development of products that sold into the growing international sleep clinic and home monitoring markets. Compumedics listed on the Australian Securities Exchange in 2000. Over the years, Compumedics has received numerous awards and accolades including Australia's exporter of the year and has been recognised as a Top 100 Innovator by both German and Australian Governments."
Founded in Australia around 1987, Compumedics Limited produces noninvasive hardware and end-user software.
Compumedics Limited makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent, Closed-Loop Efferent
Neurosensing Technique(s): EEG, MEG
Software
Hardware
- Grael 4K PSG:EEG
- Grael 4K-EEG
- Grael LT EEG System
- Neuvo 64-512 Channel LTM EEG
- Okti
- ONsight A.V.S.
- Siesta
- Xegis Forte EMG/EP
- Xegis G:NEO 2 Channel EMG/EP
FDA
Compumedics Limited has 2 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| K191785 | Magnetoencephalograph | 2 | Neurology | False | True |
| K230073 | Full-Montage Standard Electroencephalograph | 2 | Neurology | False | True |