If you're interested in becoming a contributor or requesting changes then click here to join the discord
Difference between revisions of "Philips"
Jump to navigation
Jump to search
Landonodnal (talk | contribs) |
Landonodnal (talk | contribs) |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Category:Instagram Pages]] | ||
| + | [[Category:GitHub Accounts]] | ||
| + | [[Category:LinkedIn Accounts]] | ||
| + | [[Category:YouTube Channels]] | ||
| + | [[Category:Facebook Pages]] | ||
| + | [[Category:Twitter Accounts]] | ||
[[Category:Organizations]] | [[Category:Organizations]] | ||
[[Category:Companies]] | [[Category:Companies]] | ||
| − | Philips is a health care company that focuses only on patients | + | "Philips is a health care company that focuses only on patients' proper recovery and diagnosis treatment." |
| Line 7: | Line 13: | ||
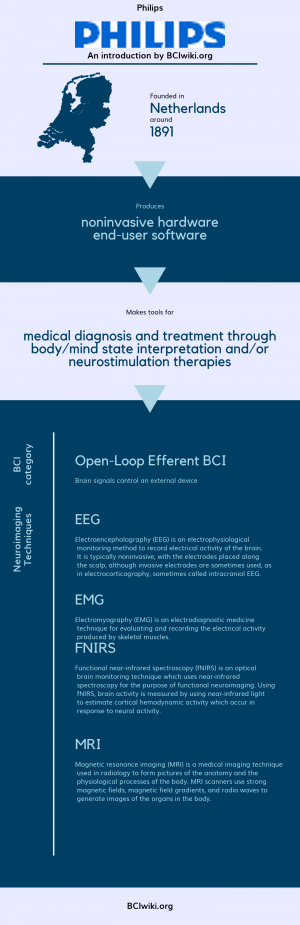
Founded in The Netherlands around 1891, Philips produces noninvasive hardware and end-user software. | Founded in The Netherlands around 1891, Philips produces noninvasive hardware and end-user software. | ||
| − | Philips makes tools for medical diagnosis and treatment through body/mind state interpretation and neurostimulation therapies. | + | Philips makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies. |
[[Brain Computer Interface Classification|BCI Categories]]: Open-Loop Efferent | [[Brain Computer Interface Classification|BCI Categories]]: Open-Loop Efferent | ||
| − | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG | + | [[:Category:Neurosensing_Techniques|Neurosensing Technique(s)]]: EEG, EMG, fNIRS, MRI |
| + | |||
| + | ==Hardware== | ||
| + | *[[CT_5100_Incisive|CT 5100 Incisive]] | ||
| + | *[[CT_6000_iCT|CT 6000 iCT]] | ||
| + | *[[Hitachi_ETG_4100|Hitachi ETG 4100]] | ||
| + | *[[Incisive_CT|Incisive CT]] | ||
| + | *[[Ingenia_1.5T_Evolution|Ingenia 1.5T Evolution]] | ||
| + | *[[Ingenia_MR-RT_XD|Ingenia MR-RT XD]] | ||
| + | *[[IQon_Elite_Spectral_CT|IQon Elite Spectral CT]] | ||
| + | *[[IQon_Spectral_CT|IQon Spectral CT]] | ||
| + | *[[MR_5300|MR 5300]] | ||
| + | *[[MR_7700|MR 7700]] | ||
| + | *[[Spectral_CT_7500|Spectral CT 7500]] | ||
| + | ==FDA== | ||
| + | Philips has 239 medical devices registered with the FDA. Here are some of them: | ||
| + | {| class="wikitable" style="margin:auto" | ||
| + | |+ Examples of FDA Approved Devices ([[Philips_FDA_Devices | View List]]) | ||
| + | |- | ||
| + | ! Device ID !! Device Name !! Class !! Category !! PMA !! PMN | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P960042 P960042] || Device, Removal, Pacemaker Electrode, Percutaneous || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P960042 P960042] || Device, Removal, Pacemaker Electrode, Percutaneous || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P180034 P180034] || Scaffold, Dissection Repair || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P960042 P960042] || Device, Removal, Pacemaker Electrode, Percutaneous || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pma&id=P180034 P180034] || Scaffold, Dissection Repair || 3 || Cardiovascular || True || False | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K201012 K201012] || System, Imaging, Pulsed Doppler, Ultrasonic || 2 || Radiology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K170144 K170144] || Interventional Fluoroscopic X-Ray System || 2 || Radiology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K171314 K171314] || System, Image Processing, Radiological || 2 || Radiology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K172406 K172406] || System, Tomography, Computed, Emission || 2 || Radiology || False || True | ||
| + | |- | ||
| + | | [https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pmn&id=K190913 K190913] || System, Image Processing, Radiological || 2 || Radiology || False || True | ||
| + | |} | ||
==Links== | ==Links== | ||
| + | |||
[https://www.usa.philips.com Website] | [https://www.usa.philips.com Website] | ||
[https://www.crunchbase.com/organization/philips-c6d9 Crunchbase] | [https://www.crunchbase.com/organization/philips-c6d9 Crunchbase] | ||
[https://www.linkedin.com/company/philips/ LinkedIn] | [https://www.linkedin.com/company/philips/ LinkedIn] | ||
| + | [https://twitter.com/PhilipsNA Twitter][https://facebook.com/Philips Facebook] | ||
| + | [https://youtube.com/Philips YouTube] | ||
| + | [https://github.com/Modernizr/Modernizr/issues/648 GitHub] | ||
| + | [https://instagram.com/philips/ Instagram] | ||
Latest revision as of 02:28, 18 April 2023
"Philips is a health care company that focuses only on patients' proper recovery and diagnosis treatment."
Founded in The Netherlands around 1891, Philips produces noninvasive hardware and end-user software.
Philips makes tools for medical diagnosis and treatment through body/mind state interpretation and/or neurostimulation therapies.
BCI Categories: Open-Loop Efferent
Neurosensing Technique(s): EEG, EMG, fNIRS, MRI
Hardware
- CT 5100 Incisive
- CT 6000 iCT
- Hitachi ETG 4100
- Incisive CT
- Ingenia 1.5T Evolution
- Ingenia MR-RT XD
- IQon Elite Spectral CT
- IQon Spectral CT
- MR 5300
- MR 7700
- Spectral CT 7500
FDA
Philips has 239 medical devices registered with the FDA. Here are some of them:
| Device ID | Device Name | Class | Category | PMA | PMN |
|---|---|---|---|---|---|
| P960042 | Device, Removal, Pacemaker Electrode, Percutaneous | 3 | Cardiovascular | True | False |
| P960042 | Device, Removal, Pacemaker Electrode, Percutaneous | 3 | Cardiovascular | True | False |
| P180034 | Scaffold, Dissection Repair | 3 | Cardiovascular | True | False |
| P960042 | Device, Removal, Pacemaker Electrode, Percutaneous | 3 | Cardiovascular | True | False |
| P180034 | Scaffold, Dissection Repair | 3 | Cardiovascular | True | False |
| K201012 | System, Imaging, Pulsed Doppler, Ultrasonic | 2 | Radiology | False | True |
| K170144 | Interventional Fluoroscopic X-Ray System | 2 | Radiology | False | True |
| K171314 | System, Image Processing, Radiological | 2 | Radiology | False | True |
| K172406 | System, Tomography, Computed, Emission | 2 | Radiology | False | True |
| K190913 | System, Image Processing, Radiological | 2 | Radiology | False | True |
Links
Website Crunchbase LinkedIn TwitterFacebook YouTube GitHub Instagram